Analyzing Delays and Enforcement Challenges in FDA Clinical Trial Reporting Oversight - An Investigation of FOIA requests
By: Megan Curtin (UAEM), Allisun Wiltshire (UAEM), Brix Kowalski (UAEM), Hanna Wu (UAEM, Johns Hopkins University), Marianne Djigo (UAEM, McGill University), Maximilian Siebert (UAEM, Stanford University)
Poster Presentation originally presented at the CERSI Scientific Symposium on January 7, 2024.
Over 4,000 (23%) of applicable clinical trials (ACTs1 as defined by 42 CFR 11.10) on the ClinicalTrials.gov database are missing results due to reporting deficiencies2. The FDA Amendments Act of 2007 (FDAAA) requires results reporting of all clinical trials funded by NIH to be submitted to ClinicalTrials.gov within 12 months of the primary completion date (42 CFR 11.44)3. FDAAA aims to ensure patient and physician access to clinical trial results, prevent scientific fraud, and avoid research duplication.
Pre-Notices are letters issued to trial sponsors who do not register an applicable clinical trial or submit required clinical trial information. The FDA will investigate cases of unaddressed Pre-Notices and potentially send a Notice of Noncompliance. If ACT sponsors do not address Notices within 30 days, the FDA may levy civil monetary penalties. However, FDA has not yet levied a single fine against a noncompliant trial sponsor.
The FDA’s risk-based approach to monitoring clinical trials is designed to “ensure the rights, safety, and welfare of participants in the clinical investigation, and guarantee the integrity of data submitted to the FDA.”4 This guidance is intended to highlight recommendations the FDA intends to follow in its monitoring and issuance of Pre-Notices and Notices of Noncompliance. Universities Allied for Essential Medicines (UAEM) is a nonprofit health equity organization.
UAEM’s Clinical Trial Transparency Campaign has been actively involved in investigating how regulators enforce FDAAA and potential mechanisms to drive clinical trial resulting reporting compliance. UAEM found in an earlier study that between 2013 and 2021 the FDA issued 57 Pre-Notices, which resulted in over 90% compliance and in cases where Pre-Notices were ignored by sponsors, 4 Notices of Noncompliance were issued in the same timeframe. While underutilized, these Notices of Noncompliance yield high compliance.
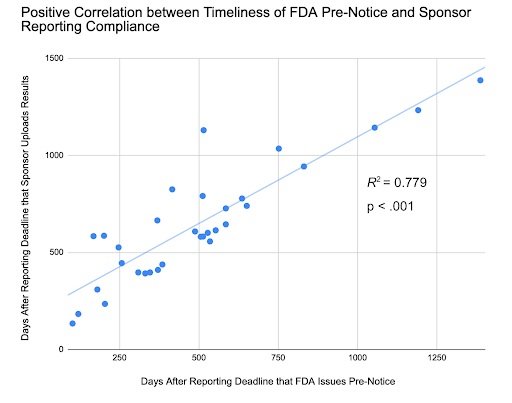
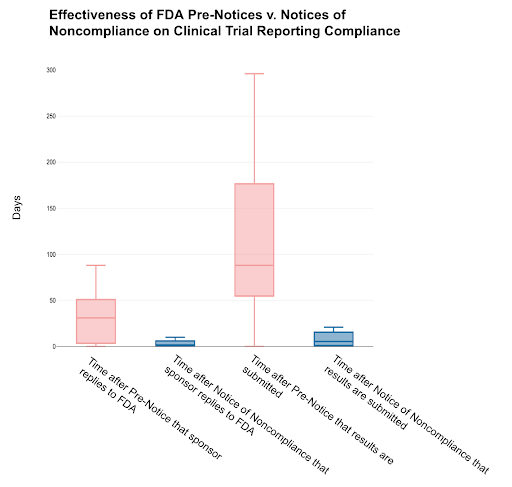
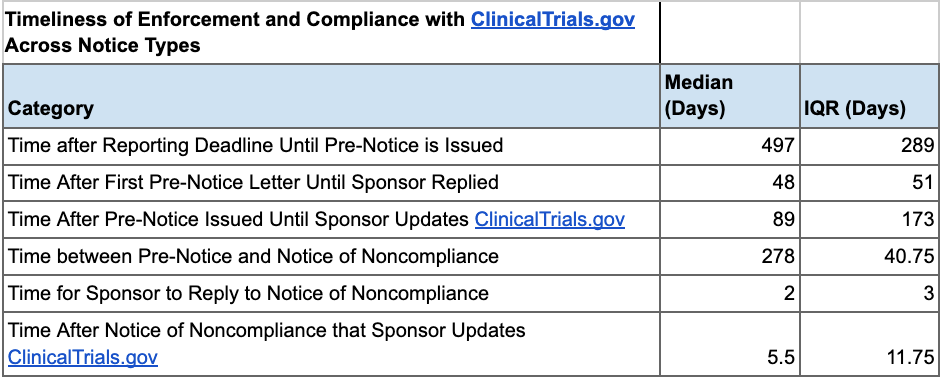
In our analysis, we found a strong positive correlation between the timeliness of FDA Pre-Notice issuance and sponsor compliance timeframes. As such, if the FDA sent Pre-Notices with greater expediency, noncompliant ACT sponsors would likely sooner address data submission issues. We also found that Pre-Notices are effective in improving compliance, yet Notices of Noncompliance yield faster responses and submission of results to ClinicalTrials.gov. These insights suggest that the FDA should increase the issuance of Notices of Noncompliance and Pre-Notices to promote greater transparency in results reporting.
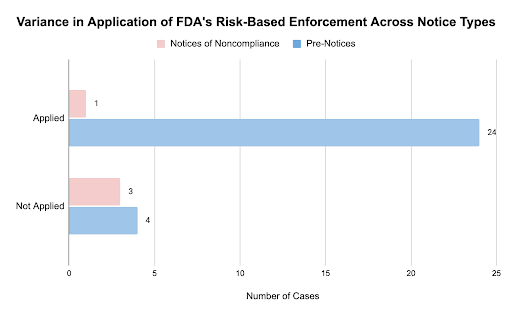
Regarding the FDA’s application of a risk-based approach, we found inconsistencies in utilization between notice types. While the majority of cases appeared to follow the FDA’s risk-based approach, 22% of Pre-Notices and only one of the Notices of Noncompliance issued were for trials that closely met the FDA’s qualifying criteria. Among the Notice of Noncompliance cohort, one study included the treatment of Acne Rosacea. These findings suggest that the FDA could allocate limited resources to prioritize enforcement for trials that pose greater risk to patients.
Our analysis was limited to the 32 cases obtained via FDA FOIA requests. In December 2023, shortly after our analysis was conducted, the FDA published all Pre-Notice letters sent, with the most recent ones having been sent out in September of this year. A future study could analyze these cases to determine how the FDA addresses outstanding ClinicalTrials.gov Quality Control Review issues and to evaluate changes in sponsor compliance patterns over time.
